Surfactant-Mediated Solubilization of Myelin Figures: A Multistep Morphological Cascade, Daniel J. Speer, James C. S. Ho, Atul N. Parikh, Langmuir 38, 8805-8816 (2022)
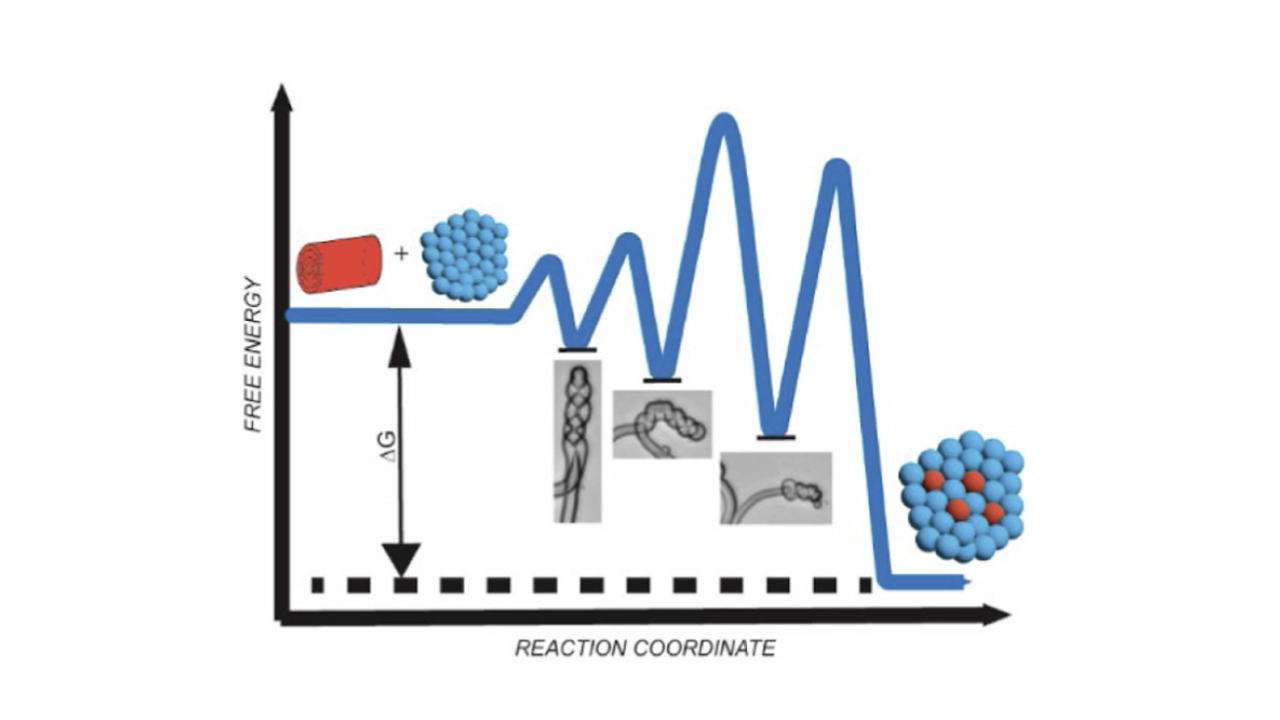
Lamellar mesophases of insoluble lipids are readily solubilized by the micellar mesophases of soluble surfactants. This simple process underscores a broad array of biochemical methodologies, including purification, reconstitution, and crystallization of membrane proteins, as well as the isolation of detergent resistant membrane fractions. Although much is now known about the thermodynamic driving forces of membrane solubilization, the kinetic pathways by which the surfactant alters vesicular mesophases are only beginning to be appreciated. Little is known about how these interactions affect the solubilization of more complex, multilamellar mesophases. Here, we investigate how a common zwitterionic detergent affects the solubilization of a smectic, multilamellar, cylindrical mesophase of lipids, called the myelin figure. Our results reveal that myelin solubilization occurs in a multistep manner, producing a well-defined sequence of morphologically distinct intermediates en route to complete solubilization. The kinetic processes producing these intermediates include (1) coiling, which encompasses the formation, propagation, and tightening of extended helices; (2) thinning, which reflects the unbinding of lamellae in the smectic stacks; and (3) detachment or retraction, which either dissociates the myelinic protrusion from the source lipid mass or returns the myelinic protrusion to the source lipid mass -all in transit toward complete solubilization. These occasionally overlapping steps are most pronounced in single-lipid component myelins, while compositionally graded multicomponent myelins inhibit the coiling step and detach more frequently. Taken together, the appearance of these intermediates during the solubilization of myelins suggests a complex free-energy landscape characterizing myelin solubilization populated by metastable, morphological intermediates correlated with locally minimized changes in energy dependent upon the mesophase's composition. This then predicts the accessibility of structurally distinct, kinetic intermediates -such as loose and tight coiled helices, peeled myelins, retracted tubes, and detached protrusions -before reaching the stable ground state corresponding to a dissolved suspension of mixed surfactant-lipid micelles.
DOI: 10.1021/acs.langmuir.2c00774