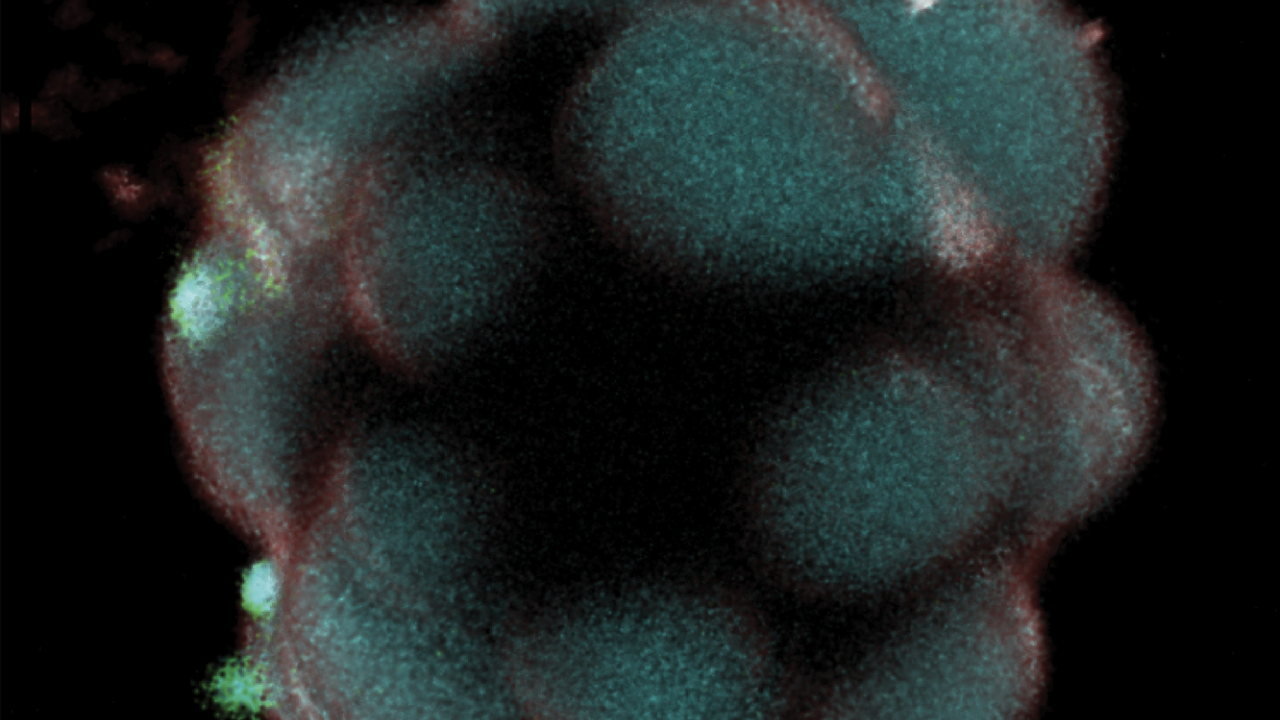
Regulating biocondensates within synthetic cells via segregative phase separation, Chang Chen, Caroline M Love, Christopher F Carnahan, Ketan A Ganar, Atul N Parikh, Siddharth Deshpande*ACS Nano 19, 20550–20563, 2025
Living cells orchestrate a myriad of biological reactions within a highly complex and crowded environment. A major factor responsible for such seamless assembly is the preferential interactions between the constituent macromolecules, that can drive demixing to produce coexisting phases and thus provide dynamic intracellular compartmentalization. However, the way multiple-phase separation phenomena, occurring simultaneously within the cytoplasmic space, influence each other is still largely unknown. Here, we show that the interplay between segregative and associative phase separation within cell-mimicking confinements can lead to rich dynamics between multiple phases and the lipid boundary. Using on-chip microfluidic systems, we encapsulate the associative and segregative components and externally trigger their phase separation within cell-sized vesicles. We find that segregative phases create microdomains and tend to dictate the fate of associative components by acting as molecular recruiters, membrane-targeting agents, and initiators of condensation. The obtained multiphase architecture provides an isolated microenvironment for condensates, restricting their molecular communication as well as diffusive motion, and can further lead to global shape transformation of the confinement itself in the form of wetted, hierarchical domains at the lipid membrane. In conclusion, we propose segregative phase separation as a universal condensation regulation strategy by managing their molecular distribution, process initiation, and spatial localization, including membrane interaction. The presented interplay between the two phase separation systems suggests a distinct design principle in constructing complex synthetic cells and controlling the behavior of artificial membraneless organelles within.
DOI:10.1021/acsnano.4c18971